

AquaTru®
Water Quality Test Kits
GENERAL
INFORMATION
Kordon's AquaTru Test Kits were designed for accuracy with
several important criteria in mind:
1-Dry,stable reagents
2-Dated reagents
3-Accurate, translucent color comparators
4-Comprehensive instructions
5-Measurement ranges that are correct for aquarium use.
 AquaTru Water Quality
Test Kits pioneered the use of dry
AquaTru Water Quality
Test Kits pioneered the use of dry
reagents for use in aquarium water testing. The major
advantage of dry reagents is in their shelf life.
Dry reagents are more stable than liquid reagents,
which means the aquarist can perform tests with the
confidence of knowing the results will be as accurate as
possible when using a "colorimetric" type test.
Kordon also dates their reagents. All manufacturer's
reagents, whether dry or liquid, are subject to degradation.
Because the reagent chemicals break down over time, it is
important to know the age of the reagents and their
expected life.
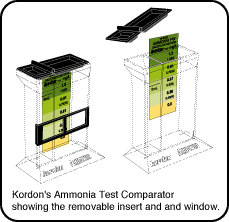
AquaTru's color comparator system has been carefully designed to
reflect the needs of the
concerned aquarist. The top is flared to make adding reagents
easier, the wide base increases
stability and a special sliding window helps make color
comparisons more accurate.
The color strip consists of translucent colors applied to a
durable plastic, rather than
a printed paper card. The translucent color strip allows light to
pass through,
providing a much more accurate match when comparing the cube's
colors to those
produced by the reagents. Reflected colors, such as those
produced on a printed comparator
do not match well when compared with the translucent colors in a
test cube.
The quality of tap water varies widely and aquarium systems are
becoming increasingly
sophisticated (and expensive). It is increasingly important to
monitor water conditions carefully
in the aquarium. It is equally important to select only top
quality, reliable test equipment to
monitor these conditions. It is beyond the scope of most
aquarists to analyze the accuracy of a
test kit. They must depend on the manufacturer to provide them
with accurate, dependable test
kits, and a staff available to answer questions and resolve
problems. Kordon has taken pride in
providing professional quality test kits and test kit
consultation... for over 15 years.
REAGENT AND COLOR STANDARD STABILITY
The reagents should be used before the expiration date shown on
the package. If the reagents are
to be used after the expiration date they should be checked with
an accurate standard solution to
insure the reliability of the reagents. The reagents should be
stored in a cool place. The color
comparator strip should not be exposed to strong light sources
for an extended length of time to
prevent fading of the colors.
Available
Test Kits:
Item Number |
Product Description |
| 35800 | Aquarist Master Test
Kit - FW & SW Ammonia (Salicylate), Nitrite, LR Nitrate, LR pH & Marine pH |
| 35810 | Fresh Water
Master Test Kit - FW Ammonia (Sal),
Nitrite, HR Nitrate & HR pH |
| 35820 | Salt Water
Master l Test Kit - SW Ammonia (Nessler),
Nitrite, LR Nitrate & Marine pH |
| 35830 | Salt Water
Master ll Test Kit - SW Ammonia (Salicylate),
Nitrite, LR Nitrate & Marine pH |
| 35840 | Low Range pH Test Kit (5.5-7.5) |
| 35860 | High Range pH Test Kit (6.5-8.5) |
| 35880 | Marine pH Test Kit (7.8-8.6) |
| 35910 | Ammonia (Nessler) Test Kit (0-3.0 mg/L) |
| 35920 | Nitrite Test Kit (0-.75 mg/L) |
| 35930 | High Range Nitrate Test Kit (0-.175 mg/L) |
| 35940 | Low Range Nitrate Test Kit (0-50 mg/L) |
| 35960 | Chelated Copper Test Kit (.5-2.5 mg/L) |
| 35970 | Ammonia (Salicylate) SW Test Kit (0-.8 mg/L) |
| 35980 | Ammonia (Salicylate) FW Test Kit (0-1 mg/L) |
| 35990 | Copper Ion Test Kit (.05-.25 mg/L) |
AquaTru®
Ammonia (salicylate)
Test Kits
Item No. 35980 (fresh water); Item No. 35970 (Marine)
GENERAL
INFORMATION
Ammonia is a principal excretion product of fishes which results
from the metabolism of
nitrogenous (nitrogen containing) compounds in their food. These
compounds are composed
mainly of protein. Ammonia is also formed from the bacterial
degradation of nitrogen containing
organic materials such as decaying plant and animal matter. It is
present in solutions as both
ionized (nontoxic NH4+) and unionized (toxic NH3); the proportion
of these two forms is pH and
temperature dependent. Click to see the section titled "UN-IONIZED AMMONIA TABLES".
Increased concentrations of ammonia in aquarium water can result
in gill tissue damage, stress and
eventual death to the fish if it is not controlled. In an
established biological filter, an autotrophic
bacteria will utilize ammonia and convert it to nitrite; however
this conversion is dependent upon
environmental conditions including pH, oxygen content, and
temperature of the water. If
conditions inhibit nitrification (the conversion of ammonia to
nitrite) or if the nitrifying bacteria in
the filter have not been established, the ammonia can reach
dangerous levels in a very short period
of time. As little as 0.6 ppm total ammonia can be toxic to fish.
Although the proportion of total
ammonia that is in the toxic (un-ionized) form is pH and
temperature dependent, it is necessary to
accurately monitor the total ammonia present so that the actual
concentration of the toxic form can
be determined.
ADVANTAGES
1) Fast and accurate readings in ranges appropriate to fish
health. Graduations of concentration
are very small to facilitate accurate readings of highly toxic
ammonia.
2) Highly stable powdered reagents. Sealed in foil pillows and
dated to insure freshness.
3) Easy-to-use kit: simple step-by-step instructions and ex-planations.
4) Readings of ammonia are expressed both as ammonia nitrogen and
as ammonia.
INTERFERENCES
Excessive levels of calcium, magnesium and nitrite in the water
will interfere in the performance of
this test when concentrations exceed 1000 mg/L as CaCO3; 6000 mg/L
as CaCO3;
and 12 mg/L NO2-N, respectively. Sulfate, nitrate and phosphate
may interfere if concentrations
exceed 300 mg/L SO-2; 100 mg/L NO3; (N) and 100 mg/L PO4-3(P).
GUIDELINES FOR ACCURATE TEST RESULTS
1) Rinse the color cube 2-3 times before collecting samples. It
is suggested that tap water be
used for the initial rinse so chemicals do not get into the
aquarium or pond water when the cube
is dipped. Aquarium or pond water should be used for final
rinsings so that residual tap water
will not affect the readings. Discard the rinse water each time
so that residual chemicals are not
poured into aquarium.
2) When collecting water samples, take care to obtain a
representative sample. For example,
collection near the bottom where organic compounds have
accumulated can result in higher
readings. The collection of water at the surface can also give
erroneous results since surface
temperature fluctuations can change the actual concentration of
the ammonia in solution.
To check sampling techniques, two samples can be taken and tested;
any variation in the results
indicate that more care should be exercised when collecting
samples. A recommended method is
to (a) submerge the rinsed, capped cube to midwater, (b) open the
cube and allow to fill with
water, (c) cap while still submerged, and (d) drain water to
correct level (discard excess).
3)Test the water sample immediately after collection.
4) For consistent accurate readings when making comparisons, use
white paper as a background
and read into normal daylight or cool white fluorescent light
sources.
DIRECTIONS FOR USE
1. Cut open one of the foil pillows marked "ammonium
salicylate" and empty contents into the
smaller cell. Put the cap on securely. Do not place your finger
over the cube's sample cell unless
the cap is in place. Shake cube several times to dissolve powder
completely. Allow sample to
set for not less than three minutes.
2. Open cap. Cut open one of the foil pillows marked "ammonium
cyanurate" and add contents
to the smaller cell. Replace the cap securely and shake cube to
dissolve completely. Allow sample
to set for 15 minutes.
3. Match the color of the sample to the closest color in the Test
Cube by moving the sliding
window up or down until both left and right windows match as
close as possible. Read the level
of total ammonia in mg/L from the scale. Flush reacted water
sample down the drain. DO NOT
return water to aquarium or pond. All fishes have varying
tolerances to ammonia. If the
concentration approaches 0.1 ppm total ammonia nitrogen, the use
of Kordon's AmQuel®
ammonia remover will reduce toxic levels.
CAUTIONS
This kit contains potentially harmful chemicals that can be
dangerous if misused. Read label
cautions carefully and exercise extreme care during handling, use
and disposal of these chemicals.
This kit should not be used by children except under adult
supervision. Following use,
always clean hands, test apparatus, and test area.
REAGENT AND COLOR STANDARD STABILITY
The reagents should be used before the expiration date shown on
the package. If the reagents
are to be used after the expiration date they should be checked
with an accurate standard
solution such as Kordon's Test Kit Standards, to insure the
reliability of the reagents. The
color comparator strip should not be exposed to strong light
sources for an extended length
of time to prevent fading of the colors. The reagents should be
stored in a cool place.
PRODUCT SPECIFICATIONS
The Freshwater and Saltwater Kordon Ammonia Test Kits are
available separately,
or in a Master Test Kit.
The Individual Test Kit includes:
A color comparator of molded plastic with a colored plastic
insert that reads 0.0-0.8 mg/L (ppm)
total ammonia in 0.2 increments (Salt Water) and 0.0-1.0 mg/L (ppm)
total ammonia in 0.2
increments (Fresh Water); 20 pillows (10 each of Ammonium
Salicylate and Ammonium
Cyanurate) sufficient for 10 tests. Detailed instructions for
each test are included in every kit.
PACKAGING:
| Item Number | Product Description |
| 35970 | Saltwater Ammonia Salicylate Test Kit |
| 35980 | Freshwater Ammonia Salicylate Test Kit |
| 35770 | Ammonia Salicylate Fresh and Saltwater Reagents (10 tests) |
| 34702 | Ammonia Salicylate Fresh and Saltwater Reagents (50 tests) |
ORGANIC
NITROGEN CONVERSIONS
A lot of confusion exists in regard to the measurement of organic
nitrogen (ammonia, nitrite and
nitrate) concentrations. When dealing with organic nitrogen in
aquariums and ponds, it is essential
to know whether readings are expressed as ion concentrations or
as nitrogen concentrations.
Many test kits do not explain how they express concentrations.
This can lead to serious
miscalculations. Ammonia concentrations in Kordon's Salicylate
Ammonia Test Kits are in units
of ammonia ion (printed in bold face) and ammonia nitrogen (see
explanation below). Giving both
methods of determining ammonia concentration allows the aquarist
to use the method that
matches the information available
Ammonia nitrogen is that nitrogen present in water that is from
total ammonia in the solution.
To convert readings of ammonia nitrogen to ammonia ion values:
Multiply the nitrogen reading by 1.3.
For example, an ammonia nitrogen concentration of .5 ppm
multiplied by 1.3 would equal .65 ppm
ammonia ion. The conversion factor of 1.3 is based upon weight
proportions of the nitrogen and
hydrogen in ammonia (1.3 grams of ammonia contain one gram of
nitrogen).
The calculation of the conversion factor is as follows:
Nitrogen atomic weight: 14.01
Hydrogen atomic weight: 1.01
Molecular weight NH4 (ammonium):
(M.W. nitrogen) + (4) (M.W. hydrogen) = (14.01 + (4) (1.01) =18.05
Ammonia nitrogen = 14.01 / 18.05 = 0.7762
Click here to return to AQUABAZ Home Page