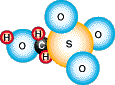
sodium hydroxymethanesulfonate, HOCH2SO3Na.
The active part of the molecule can be graphically represented as:
AmQuelŽ
How It Works
AmQuel was the first,
and is still the safest and most reliable substance for
controlling ammonia
and chloramines in aquariums and ponds. Before AmQuel came along,
aquarists had to use
fairly complicated methods for controlling ammonia in fresh water,
and its control in salt water
remained a problem.
The active
ingredient in AmQuel is known chemically as 
sodium hydroxymethanesulfonate, HOCH2SO3Na.
The active part of the molecule can be graphically represented as:
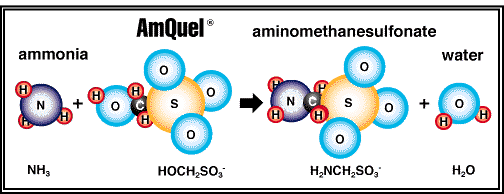
The hydroxymethane-
end of the molecule reacts with ammonia to form a
non-toxic, stable water-soluble substance which is acted upon by
biological filtration.
This reaction effectively removes the toxic ammonia from solution.
Even in water of low pH (<7.0)
the above reaction proceeds to completion. This is because even
at pHs below 7.0 there is always
some "free" ammonia (NH3) and the AmQuel will scavenge
it from the water. This is why AmQuel
works faster at higher pH's and in saline waters.
The substance formed
is stable, and testing has shown that even after weeks in an
aquarium
without a biological filter, the ammonia is not released back
into the water. Also, unreacted
AmQuel is stable, and unless removed with water changes or
granular activated carbon it will be
available to react with ammonia until it is exhausted in the
water to which it was added.
This is why AmQuel has proven so useful in shipping fishes.
The -sulfonate end of the AmQuel molecule reacts with both free-available
chlorine, known
properly as hypochlorites (OCl-) and combined-available chlorine
(chloramines). In the first
instance nothing more than harmless chloride ions (Cl- ) are
produced, and in the latter instance
chloride ions are formed and the freed ammonia instantly reacts
with the hydroxy-methane end
of the molecule.
For additional information on AmQuel, click here: AmQuel
U.S. Patent No. 4666610
European Patent No. 0203741
Canadian Patent No. 1300286
Japanese Patent 1850992
Click here to return to AQUABAZ Home Page