to review the following information to help better understand what ammonia test kits and their
readings are all about. First, you should know that what is generally referred to as "ammonia"
is in two forms: un-ionized ammonia (NH3) and ionized ammonia (NH4+). Ionized ammonia
is relatively nontoxic while un-ionized ammonia is toxic to fishes and aquatic invertebrates,
even in low concentrations.
Ammonia test kits commonly available in the aquarium pet industry read total ammonia: which
include both unionized and ionized ammonia (NH3+NH4+). The unionized (toxic) form of
ammonia is a part of the total reading, and two determinations have to be made to find the
amount that is in the toxic form. Consult the paragraph entitled "The effects of pH and temperature
on ammonia" at the end of this article for additional information on toxic ammonia levels.
There are two kinds of test kit readings; one for ionic ammonia and the other for ammonia nitrogen
(a reading of the nitrogen present in NH4+). Some pond and aquarium test kits give readings as
ammonia nitrogen, some as total ionic ammonia, and many don't explain how their readings are
calculated. Older Kordon test kits read in units of total ammonia as nitrogen (N) for the salicylate
kits and as ammonia ion for the nessler kit (as indicated in the instructions supplied with each kit).
The latest generation of Kordon's ammonia tests kits read both the ion and as nitrogen.
What is the difference between test kit readings of ammonia ion and ammonia/nitrogen?
Many aquarists and pond keepers are perplexed by the use of the terms ammonia ion and
ammonia/nitrogen (or ammonia as nitrogen), and do not know what the difference is between
the two. The differences between the two terms are in how the chemical composition of the same
ammonia molecules are being measured, which can be in two different ways, giving two different
readings, each of which is correct. If you have a reading specified as ammonia/nitrogen it can be
converted to ammonia ion by multiplying the reading by 1.3. If the reading is expressed as
ammonia ion, it can be converted to ammonia/nitrogen by dividing by 1.3. For example, if the
ammonia ion reading is 2.6 ppm, you can divide by 1.3 and you will get an ammonia/nitrogen
reading of 2.0 ppm (2.6 / 1.3= 2.0). The conversion factor of 1.3 is based on the atomic weight
proportions of nitrogen and hydrogen in ammonia (1.3 weight units of ammonia contain 1.0
weight units of nitrogen).
If the ammonia is measured as the total molecular weight of the molecules of the ammonia
compounds in the water, which will be NH3 + NH4+, the total amount of combined nitrogen
and hydrogen atoms in the molecules are being measured as ammonia ion. If only the nitrogen
atoms contained in the complete ammonia molecules are being measured and the measurement
for the hydrogen atom is left out, the reading is for ammonia/nitrogen (N).
Most information in the aquarium and pond keeping hobby refers to ammonia as the total ionic
ammonia so when you see the reference to ammonia as 0.8, for example, this usually means in
aquarium and pond publications the total molecular weight for all the ammonia molecules. The
number 0.8 means that the total weight is in ppm (parts per million) or mg/L (milligrams per liter),
which for our purposes are essentially the same measurement. When the information provided
does not indicate whether it is as ammonia ion or ammonia as nitrogen, there indeed can be
confusion as to which is being measured. However, when you remember that the two readings
are different by 1.3x, it may be possible to know to which of the two types are involved; at least
for general aquaristic and pond keeping purposes the readings are close enough together that it
is not of major concern.
Why are there two different ways to refer to measuring the amount of ammonia in the water?
In scientific measurements of water quality in lakes, streams and oceans, the amount of nitrogen
in the water, what ever the form, is an important measurement. Therefore, ammonia as nitrogen
is widely used in the scientific literature. In biological laboratory research on living aquatic animals,
the amount of total ionic ammonia present is important to know, particularly in considering the
ammonia ions' effect on the physiological processes of the animals.Therefore, in this type of
research, and in much of aquarium and ornamental pond keeping, it has been more important to
measure ammonia as ammonia ion. But keep in mind that both readings are correct, and either
reading can be directly acquired by multiplying or dividing by 1.3, respectively.
When theammonia readings are made, what do they mean?
This question is often asked by aquarists and pond keepers wanting to know what the
significance of their readings really are. Put simply, it is important to keep the level of ammonia
as low as possible in the water, because it is toxic to fishes and aquatic invertebrates (toxic
means poisonous; stressful but not necessarily lethal) . Even levels below 0.5 ppm (0.5 mgL)
are toxic or enfeebling to many aquatic animals. To immediately get rid of the ammonia in the
water use Kordon's AmQuel® (which quickly and permanently combines with the ammonia
molecules to form new, non-toxic molecules, see AmQuel), or make partial or whole water
changes. Over time, a properly set up biological filter will control ammonia build up. For
specific aquatic animals check the technical aquaristic literature for their tolerance to ammonia.
In general: Many of the hardiest fishes cannot survive toxic ammonia levels above 3.0 ppm;
sick fishes succumb at lower toxic ammonia levels than healthier fishes; most marine animals
have a much lower tolerance to ammonia than freshwater animals, although many crustaceans
(crabs, shrimp, lobsters) can stand 9.0 ppm and higher levels of toxic ammonia. To keep on the
safe side use Kordon's AmQuel or do water changes as soon as you see 0.2 ppm or more total
ammonia in the water.
Where does the ammonia come from?
Uneaten food, dead and decaying animals and plants, as well as the fecal, urinary and respiratory
waste products of the aquatic animals are broken down into ammonia by heterotrophic bacteria
in a process called amonification. A secondary ammonia source is tap water. Municipal water
supplies often have high levels of ammonia added to the water combined with chlorine to
producechloramines. Chloramines are used to control bacteria and are highly toxic to aquatic
animals.
It is important for fish keepers to know whether or not chloramines are being used in their tap
water, contact the local water supplier for information. Chloramines are extremely stable and will
not "gass off" like chlorine. For information on dealing with chloramines see the AmQuel
Product Data Sheet. Whatever the source of ammonia in the water, it exists in a balance
between ionized and un-ionized, converting back and forth depending upon the pH and
temperature of the water. Ammonia (that is not combined with chlorine) is normally converted
to nitrite and ultimately nitrate by naturally occuring nitrifying bacteria. This action prevents the
buildup of potentially lethal ammonia concentrations in the water. If anything happens to the
water that is detrimental to these bacteria (low alkalinity, low oxygen level, addition of
antibiotics, etc.) the bacteria will become dormant or die, and the ammonia level in the water
will immediately increase. Therefore, monitoring of aquariums and ponds by use of water quality
test kits is very important.
What is the effect of pH (Acidity/Alkalinity) and temperature on ammonia?
If you want to be more exact in assessing whether the amount of ammonia in the water is in the
toxic form or not, it is necessary to measure the pH as well as the ammonia, because the pH of
the water determines how much of the ammonia is in the un-ionic (toxic) form. Dependent upon
the pH of the water, the ratio between ionized (nontoxic) and un-ionized (toxic) ammonia changes
rapidly. The ammonia molecules are in balance between the nontoxic ionized and the toxic
un-ionized; the higher the pH the more ammonia is in the toxic form; conversely, the lower the
pH the less ammonia is in the toxic form. Temperature also plays a part in determining how much
of the total ammonia molecule is in the toxic form; the higher the temperature, the more toxic the
ammonia.
Kordon has created a chart of ammonia tables that show how to determine the amount of toxic
ammonia in a given sample of water when the pH and temperature of the water and the amount
of total ammonia are known. Once test kit readings are made of the ammonia, pH and
temperature, the exact amount of ammonia in the toxic form can be determined. This chart also
points out how pH and temperature can have a dramatic effect on the amount of toxic ammonia
present in an aquarium or pond. For example, if the ammonia reading is 0.5 ppm (mg/L), if the
pH is 7.0 and if the temperature is 73.4° F then the amount of toxic ammonia is .0025; the amount
of toxic ammonia in this sample is below a harmful level for most aquatic animals. If only the pH
were raised to 8.0, the amount of toxic ammonia would also rise to .0218 ppm (mg/L) which is
a harmful level for many aquatic animals. This result would indicate that Kordon's AmQuel should
be added to the water to eliminate the toxic ammonia, or that a water change should be made.
Testing should be continued to be sure that harmful levels of toxic ammonia do not recur.
Click here for access to the AMMONIA TABLES.
When using colorimetric tests kits, including pH, keep in mind that many have, at best, an
accuracy range up to plus or minus 10%. Kordon's Aqua·Tru Test Kits are accurate within
plus or minus 5%.
Why are there two kinds of ammonia test kits, NESSLER and SALICYLATE?
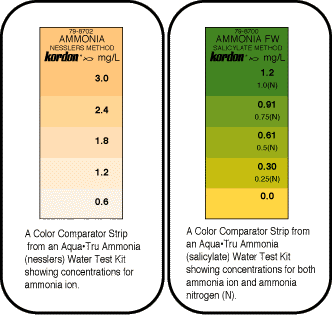
Kordon's original colorimetric test kit
used the nessler method, which
measures in shades of amber from
0.6 to 3 ppm. Its advantages are that
it reads a wide range of ammonia, and
the test is fast to perform.
The main disadvantages are the difficultes
encountered when determining low levels
of ammonia and the fact that the steps
are farther apart than those in the salicylate
method. Most ornamental aquarium and
pond fishes can only tolerate a few ppm of
ammonia at most and they need a test kit
mainly reading below 1 ppm.
The salicylate method test kits read
between 0 and 1 ppm, the ideal range for
ornamental pond and aquarium keeping.
The colors range from a bright yellow (no ammonia) through various shades of green to indicate the presence of ammonia; these colors are much easier to read than similar nessler's method test kits.
Because of the way in which AmQuel reacts with organics, salicylate test kits are the only
type suitable for use with AmQuel. The reagent used in nessler method test kits will react with
AmQuel, producing a dark brown color, falsely indicating a high level of ammonia present in the
test sample.
Click here to return to AQUABAZ Home Page
